Biospecimens Managed
CAP,CLIA,ISO, & FDA-Registered
Trials Conducted for IVD, CDx & Therapeutics
PBMC, DNA & RNA Isolations
Countries with real-time sample processing
Our Biospecimens
Blood, Biofluids, and Derivatives
Diseased and healthy human blood, plasma, serum, CSF, stool, ascites fluid, saliva, urine and more.
Tissues
Pathologist-verified, fresh, frozen, and fixed tissue specimens from healthy and diseased human subjects.
Viable Cells
HLA-typed cellular products including PBMCs, BMMCs, Leukopaks, DTCs and more.
Remnant Diagnostic Specimens
Remnant laboratory specimens characterized by FDA-cleared and ROW assays.
Liquid Biopsy
Comprehensive services including kitting, collection, processing, & profiling from your patients or ours.
Custom Biospecimen Collections
Global clinical network, regulatory approved, and ready to enroll.
Our Solutions and Services
Biomarker-driven labs, analysis, and trials for all stages of drug and diagnostic development, backed by a responsive team of industry veterans. Learn about our array of services here at the biorepository and at our other Precision for Medicine sites.
World-Renowned Biorepository
100,000 Sq. ft. CAP-certified biorepository providing specimen processing, storage, management, logistics, and distribution of your critical research specimens.
Biomarker & Specialty Lab Services
Precision for Medicine has a global footprint of CLIA, CAP, GCP specialty labs available to generate robust biomarker data for your research projects. Explore the Precision for Medicine lab services below to learn how we can help with your scientific research and development activities.
Learn More at the Precision for Medicine website:
Regulatory Strategy
Our experts can support you through regulatory strategy and submissions for CDx, IVD, and LDTs. Our team has helped hundreds of clients gain authorizations, clearances, and approvals for their diagnostics.
- Regulatory pathway and strategy
- Global submissions including United States (EUA, 510(k), De Novo, PMA), European Union (CE Mark to IVDD and IVDR), Japan (PMDA), Health Canada, and more, applied to traditional in vitro diagnostics, LDTs, or CDx
- Presubmission package development
- Quality systems implementation and oversight
Commercial Consulting
Our team of experienced market access and reimbursement strategists can help you develop and execute on your go-to-market strategy.
- Market access and channel optimization
- Coding and payment strategy and support
- Assay platform and format selection
- Utility demonstration and access to coverage
Study Specimen Collection Kits
Study-specific specimen collection kits designed to simplify enrollment and maximize your specimen and data quality.
Clinical Trial Services and Management
End-to-end CRO services for your biomarker-driven clinical trials.
Learn More at the Precision for Medicine website:


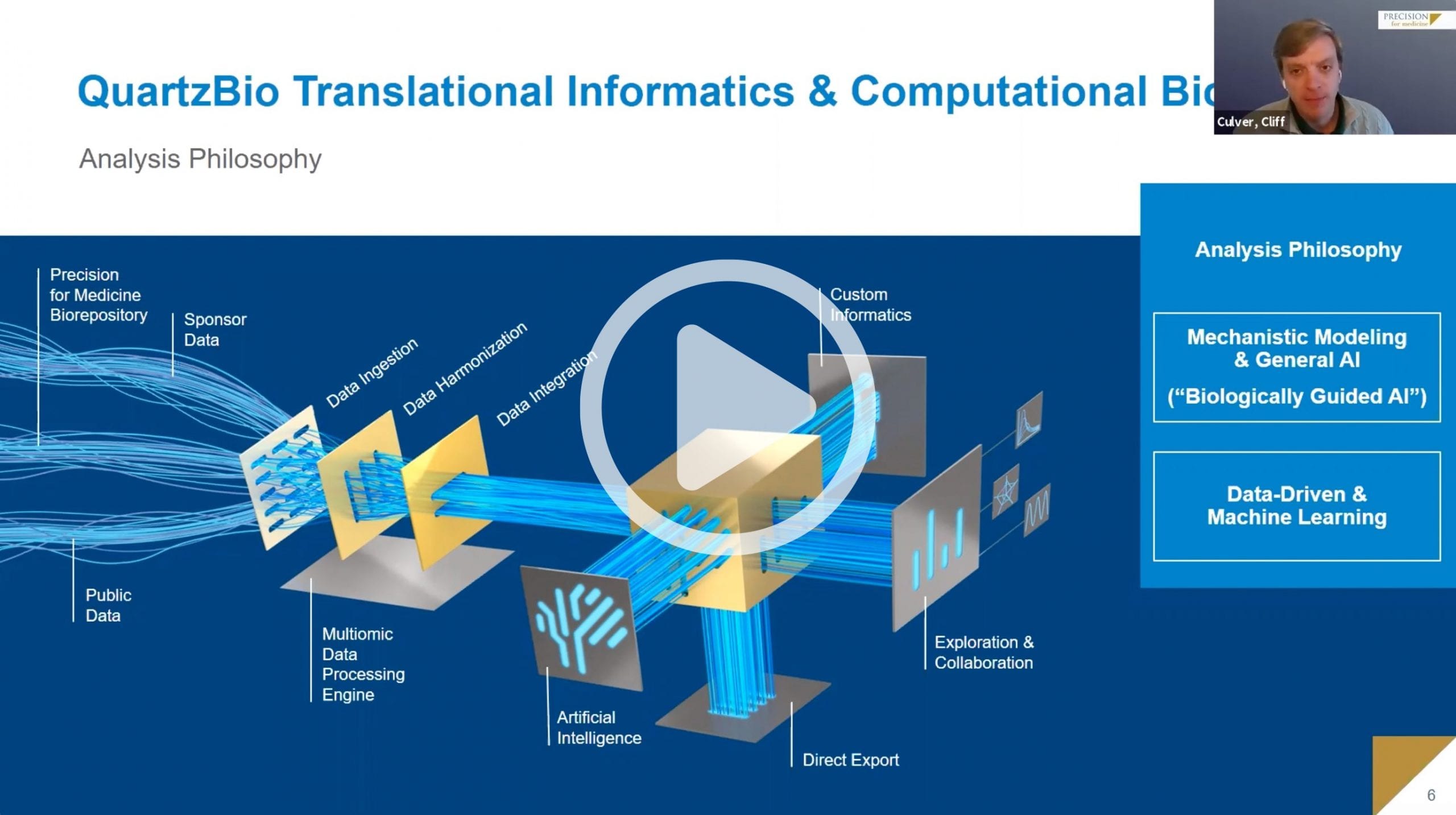
Data Science
Data aggregation, harmonization, and analysis for your biomarker program leveraging our proprietary QuartzBio platform and team.
Learn More at the Precision for Medicine website: